How Many Orbitals in the 6p Sublevel
Three orbitals in the p-subshell. How many orbitals can n 4 Class 11 have.
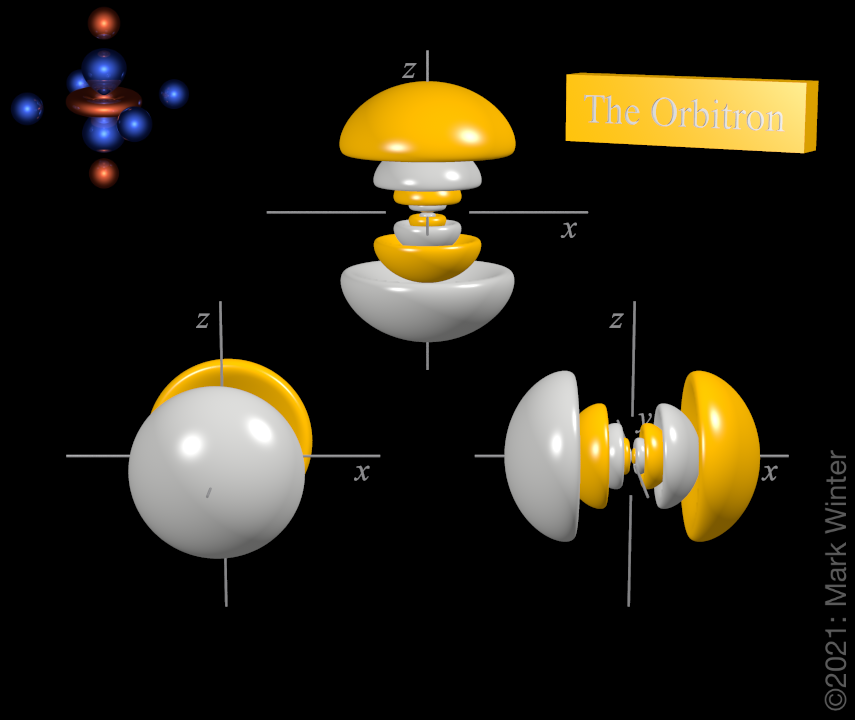
The Orbitron 6p Atomic Orbitals
Click card to see definition.
. There are four types of. Each orbital holds two electrons for a total of six electrons. So the n 6 shell contains three subshells particularly 6s 6p and 6d.
What would be the sublevels in n 6. Each sublevel can have 6 electrons basically 2 s orbital electrons 6 p orbitals electrons 14 f orbital electrons Hope this helps. An f sublevel has 7 orbitals.
P sublevel has 3 orbitals. If not sorry Advertisement. How many possible orbitals are there for N 4.
2nd level has 4 orbitals. The p sublevel has three orbitals and can contain 6 electrons. How many orbitals are in the 6p sublevel.
Is there a 5g sublevel. 6 in a p sublevel 18 in the 3rd level 14 in an f sublevel and 2 in one orbital 9. How many orbitals are in the 6p sublevel.
For any atom there are three 6p orbitals. There are 3 orbitals in the p level. These orbitals have the same shape but are aligned differently in space.
The f sublevel has. And the 4 sublevel has 7 orbitals so can contain 14 electrons max. Of orbitals 22 4.
These orbitals have the same shape but are aligned differently in space. List the sublevels in order from n1 to n4 overlapping in energy level with the extras added only type the ones added in 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p. There are 2l1 such values of ml for a given l and thus there are 2l1 orbitals in each subshell.
The second energy level will thus have a total of 4 orbitals. 49 5 35 votesEach orbital contains at most two electrons. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p.
These orbitals will be distributed on two subshells the s-subshell and the p-subshell. These orbitals have the same shape but are aligned differently in space. In the case of the 6d-subshell the principal quantum number n which gives you the energy level on which the electron can be found is equal to 6.
Tap card to see definition. For any atom there are three 6p orbitals. The number of orbitals in a shell is the square of the principal quantum number.
Therefore the p-sublevel has a total of three p-orbitals. These orbitals have the same shape but are aligned differently in space. When we consider one other than s the contained orbitals are defined as degenerate ie.
How many orbitals are in the 6p sublevel. Click again to see term. These orbitals have the same shape but are aligned differently in space.
How many orbitals are in the 6p sublevel. Atomic orbital - Wikipedia. The filling order of the orbitals in ascending order of energy is as follows.
One orbital in the s-subshell. The possible subshells for n6 are 6s6p6d6f6g6h and the orbitals associated with them are 1357911. What is a 6d orbital.
The orbitals are s p d and f. 3 rows For any atom there are three 6p orbitals. How do you calculate orbitals in a sublevel.
This phenomenon is due to the fact that in an atom the electrons arrange themselves occupying first the orbitals with lower energy and gradually those with higher energy. The p sublevel has 3 orbitals so can contain 6 electrons max. All p orbitals are 3 in number.
Which subshell has 5 orbitals. The fourth and higher levels also have an f sublevel containing seven f orbitals which can hold a maximum of 14 electrons. For any atom there are three 6p orbitals.
You can thus say that the second energy level will contain. Tap card to see definition. Energy Levels Energy Sublevels Orbitals Pauli Exclusion Principle.
The number of orbitals in a subshell is therefore 2 l 1. You examine the range of allowed values of ml for a given l. There is one orbital in an s subshell l 0 three orbitals in a p subshell l 1 and five orbitals in a d subshell l 2.
Px py and pz. Energy Levels Energy Sublevels Orbitals Pauli Exclusion Principle. The three 6p orbitals normally used are labelled 6px 6py and 6pz since the functions are aligned along the x y and z axes respectively.
Doriana Dangelo Last updated. Each ml value corresponds to an orbital in the subshell defined by l. For any atom there are three 6p orbitals.
12 1 22 4 32 9. Which orbital is filled immediately before the 5f orbital-Therefore we can conclude that 7s-orbital is filled just before 5f-orbital. Orbitals in 6p sublevels.
Subshell 6s entails 1 orbital subshell 6p entails three orbitals 6px 6py and 6pz and subshell 6d entails 5 orbitals 6dxy 6dxz 6dyz 6dx2-y2 and 6dz2. With a equal associated energy. 2 Orbitals are combined when bonds form between atoms in a molecule.
The d sublevel has 5 orbitals so can contain 10 electrons max. How many orbitals are in the 6p sublevel. An energy sublevel s p d f includes one or more orbitals and when a sublevel contains more than one ie.
These orbitals have the same shape but are aligned. For any atom there are three 6p orbitals. How many electrons can each orbital hold.
Tap again to see term. For n 3 there are nine orbitals for n 4 there are 16 orbitals for n 5 there are 5 2 25 orbitals and so on. The d sublevel has five orbitals and can contain 10 electrons.

6 Shells Subshells And Orbitals Youtube

Orbital Diagrams Overview Examples Expii
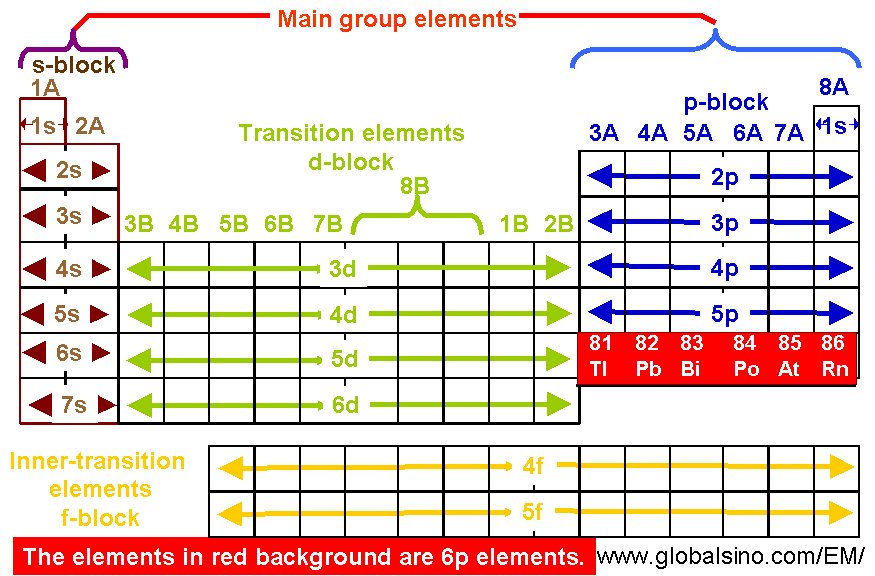
6p Elements In Periodic Table Structure Of The Periodic Table
Solved 5 Which Elements Are Represented By The Following Chegg Com

Different Strategies For Determining Electron Configurations Of Download Scientific Diagram

4 Major Groups Of The Periodic Table Geometry Worksheets Electron Configuration Mathematics Worksheets

In The Sixth Period The Orbitals Being Filled Are Youtube
Electron Configurations And Atomic Orbital Diagrams Chemistry

Electron Configurations And Orbital Diagrams Maximum Number Of Electrons In Each Sublevel Maximum Number Of Electrons In Each Sublevel Maximum Number Ppt Download
12 1 Electron Configuration Hl Chemninja
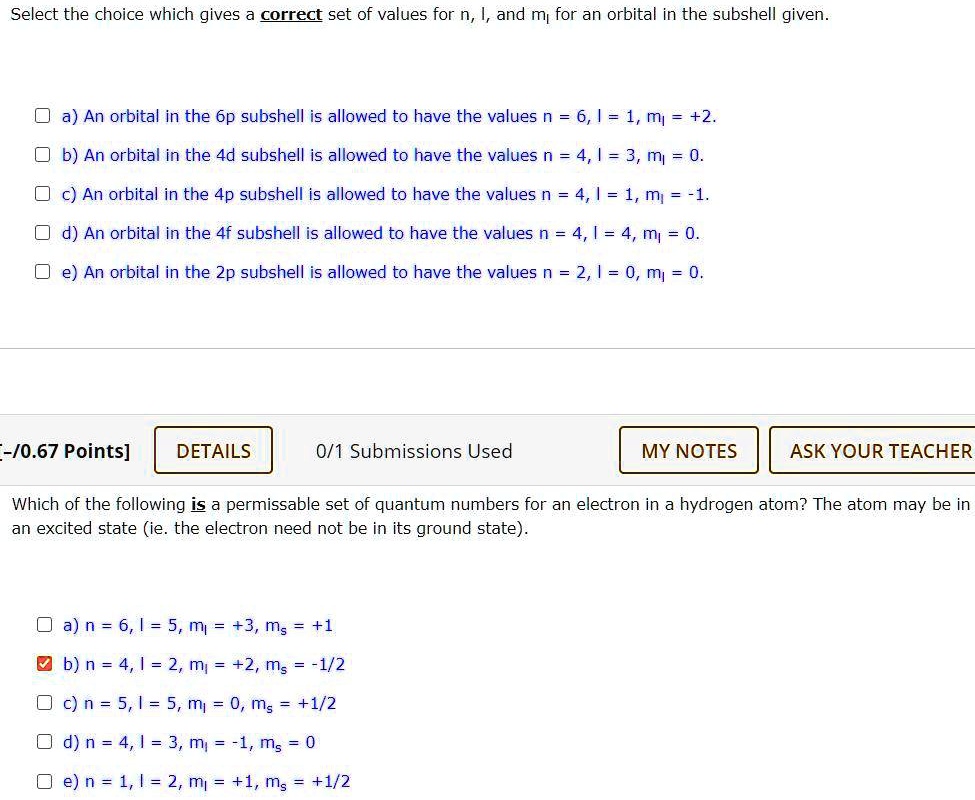
Solved Select The Choice Which Gives A Correct Set Of Values For N I And M For An Orbital In The Subshell Given A An Orbital In The 6p Subshell Is Allowed To
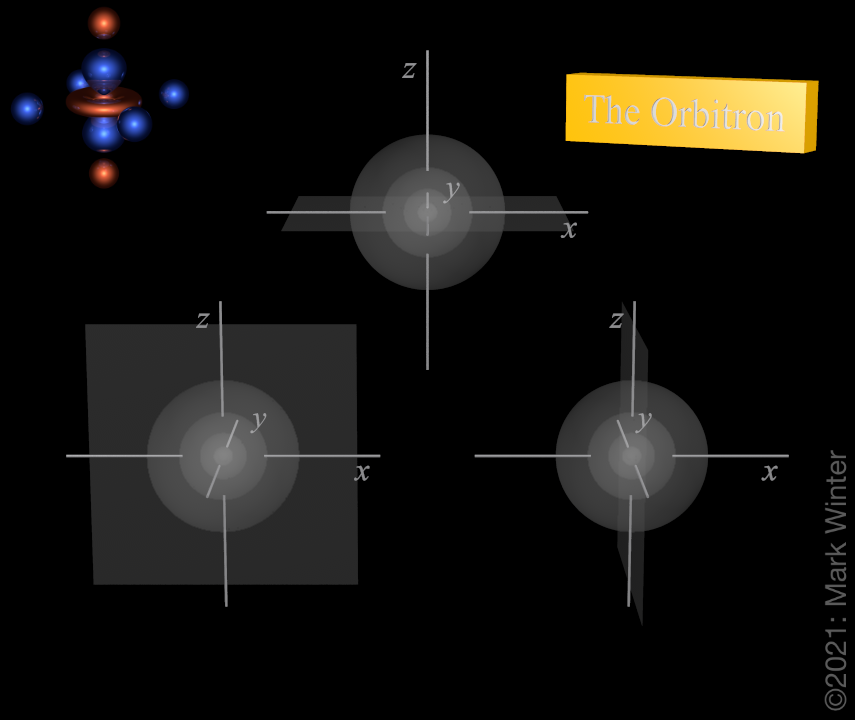
The Orbitron 6p Atomic Orbitals
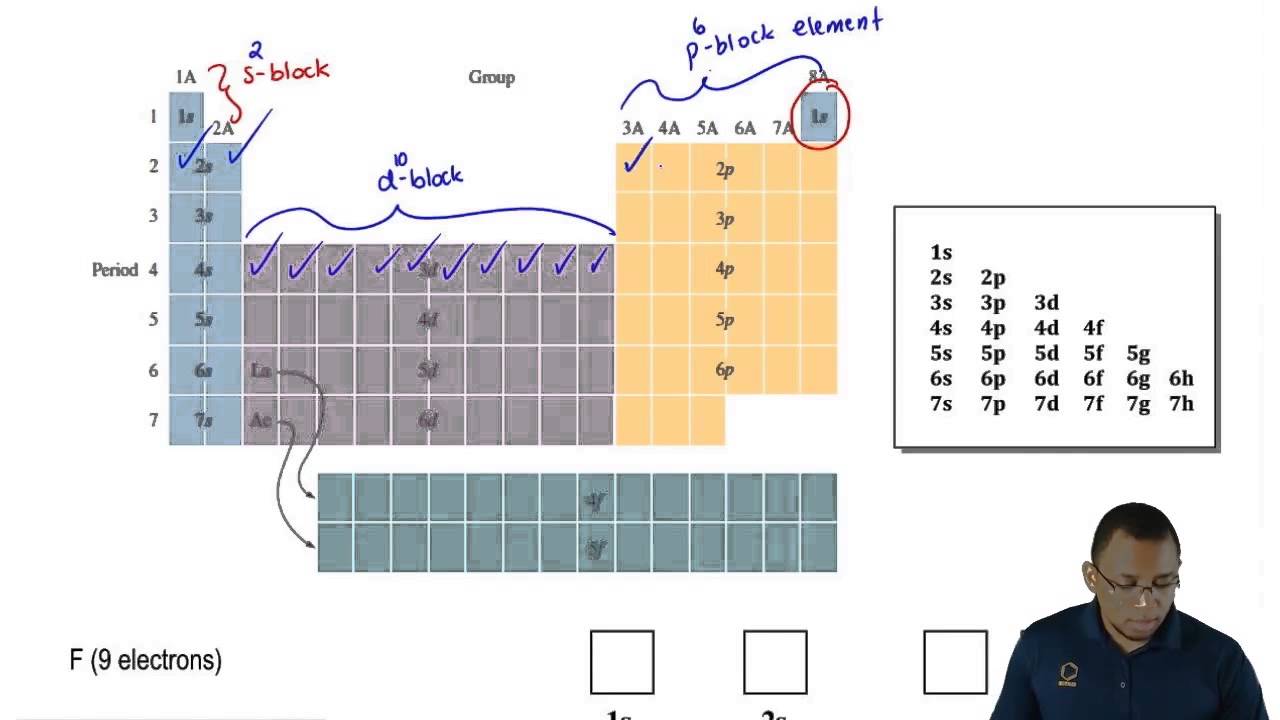
The S P D F Sublevels And The Periodic Table Youtube

Advanced Electron Configuration
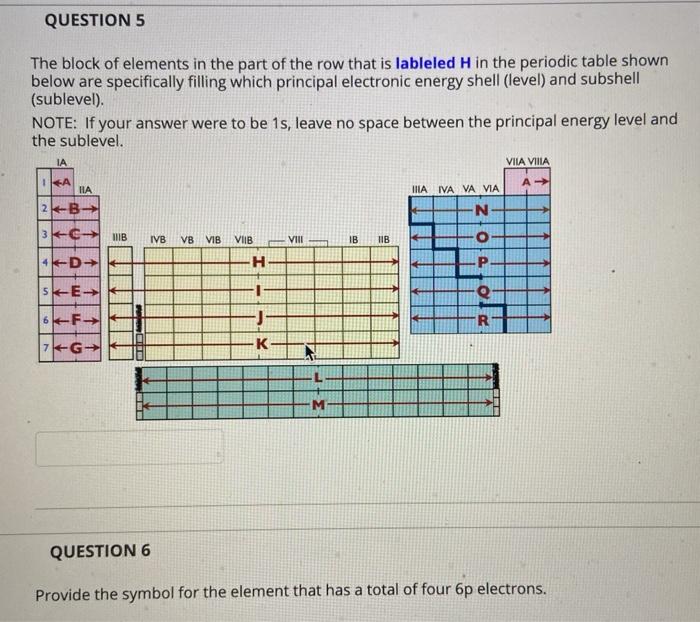
Solved Question 1 Arrange The Following Types Of Chegg Com
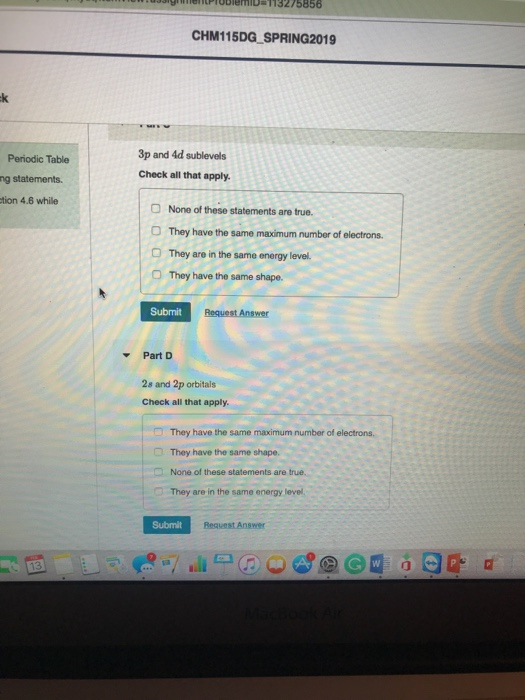
Solved Chm115dg Spring2019 Dic Table Ements 6 While 5p And Chegg Com


Comments
Post a Comment